Abstract
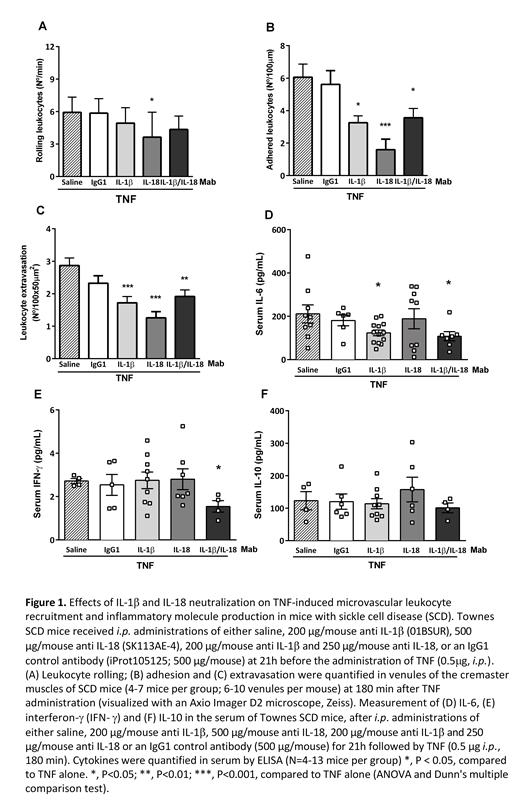
The chronic inflammatory state associated with sickle cell disease (SCD) incurs pan-cellular activation and the recruitment of leukocytes to the activated endothelium of blood vessels. The resultant rheological alterations and red cell sickling culminate in acute vaso-occlusive processes. Cytokines IL-1β and IL-18, the bio-active products of the activated inflammasome, are elevated in the plasma of SCD patients (ASH Abstract (2016) 128 (22): 854), and a previous study reported that anti-IL1β therapy alleviated reperfusion injury and flow stasis in NY1DD transgenic sickle mice exposed to hypoxia/reoxygenation (ASH Abstract (2011) 118(21): 848). The aim of this study was to determine whether antibodies that neutralize IL-1β and IL-18 could individually, or synergistically, diminish inflammatory processes and leukocyte recruitment in mice with SCD. Townes mice (5-months old; N=4-7 per group) received an i.p. administration of either saline, 200 µg/mouse anti-murine IL-1β (01BSUR), and/or 250 or 500 µg/mouse anti-murine IL-18 (SK113AE-4), or an IgG1 control antibody (iProt105125; 200 or 500 µg/mouse). At 21h after treatments, vaso-occlusive-like processes were induced in mice by the injection of tumor necrosis factor-α (TNF; 0.5μg, i.p.). At 3h after TNF, the cremaster muscles of anesthetized mice were surgically exposed, and leukocyte TNF recruitment and extravasation in venules of the microcirculation were observed using intravital microscopy. Another set of Townes mice (N=4-12 per group) was submitted to the same procedures, with blood sampling for ELISA at 3h post TNF. Optimal concentrations of antibodies were determined by observing leukocyte recruitment by intravital microscopy (doses; 100, 200 µg/mouse anti-IL-1β [N=2; 3, respectively] and 250, 500 µg/mouse anti-IL-18, [N=2 each]) in TNF-stimulated C57BL/6J mice (data not shown). Figure 1 (A-C) demonstrates the extensive recruitment and extravasation of leukocytes that occurs in the microvasculature of Townes mice at 3h post-TNF (saline group). Pre-treatment of mice with either anti-IL-1β or anti-IL-18 significantly abrogated (P<0.01) the TNF-induced adhesion (Fig. 1B) and extravasation (Fig. 1C) of leukocytes in venules, while only anti-IL-18 significantly reduced leukocyte rolling along the venule endothelium (Fig. 1A; P<0.05). In contrast, the administration of 500 µg/mouse (Fig. 1) or 200 µg/mouse (data not shown) of a non-specific IgG1 did not significantly affect TNF-induced leukocyte recruitment/extravasation (P>0.05). The combined use of the anti-IL-1β (200 µg/mouse) together with an intermediate dose of anti-IL-18 (250 µg/mouse) did not further reduce leukocyte recruitment and extravasation in this model, when compared with the effects of anti-IL-1β alone (Fig. 1A-C; P>0.05). Investigating the effects of these biological agents on inflammatory molecules production in TNF-stimulated Townes mice, we found that the administration of anti-IL-1β (200 µg) reduced IL-6 production, a pleiotropic inflammatory molecule that is upregulated by IL-1β (Fig. 1D), as did the combination of anti-IL-1β plus anti-IL-18 (200 µg and 250 µg/mouse, respectively). Pre-treatment of TNF-stimulated SCD mice with anti-IL-1β plus anti-IL-18 decreased Interferon (IFN)-γ production, a molecule that is upregulated synergistically by IL-18 and IL-12 and that mediates early host immune defenses (Fig. 1E). In contrast, anti-inflammatory IL-10 production was not significantly modulated by the pre-treatment of TNF-stimulated mice with these biological agents (Fig. 1F). As such, IL-1β and IL-18 neutralization significantly reduced inflammatory processes and leukocyte recruitment in the microvasculature of mice with SCD, indicating that biological agents that inhibit the effects of inflammasome-processed cytokines may hold potential for reducing vaso-occlusive processes in patients with this disease.
Kovarik: Novartis Institutes for Biomedical Research: Current Employment. Costa: Novartis: Consultancy. Conran: Novartis Pharma AG: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal